(-)-Talaumidin (1), a 2,5-biaryl-3,4-dimethyltetrahydrofuran lignan isolated from Aristolochia arcuata Masters, shows essential neurite-outgrowth promotion and neuroprotection in important cultured rat cortical neurons and in NGF-differentiated PC12 cells.
The first enantioselective complete synthesis of 1 was achieved by a flexible and reliable synthetic pathway involving an Evans uneven aldol response, along with a stereocontrolled hydroboration and Friedel-Crafts arylation, to assemble the Four contiguous chiral services on the tetrahydrofuran (THF) ring of 1.
In order to analysis the stereochemistry-activity relationship of 1, a scientific synthesis of all diastereomers of 1 was achieved by making use of the substitute method used for pure product 1. The evaluation of neurite-outgrowth promotion by all of the synthesized diastereomers indicated that the (-)-(1S,2R,3S,4R)-isomer 1e was significantly further full of life than naturally occurring 1.
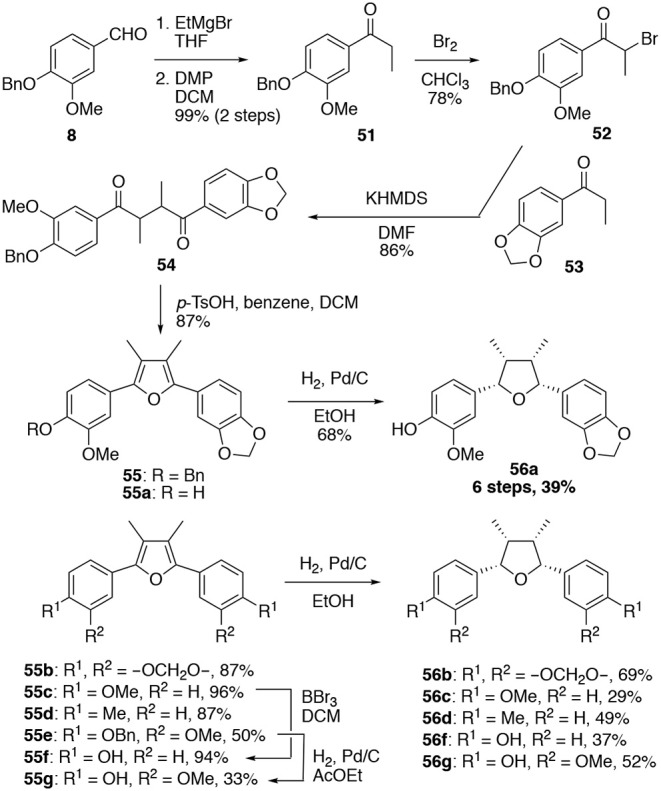
Additionally, we established a man-made methodology for talaumidin derivatives which will very nicely be used to arrange a spread of analogs in a pair of steps and on a giant scale.
The synthesized racemic analog rac–1e (56a) exhibited neurite-outgrowth promoting train in NGF-differentiated PC12 cells to the similar diploma as a result of the optically full of life (-)-1e, revealing {{that a}} relative configuration bearing all-cis– substituents is important for potent neurotrophic train, whereas completely the configuration would not affect train.
Fourteen analogs based totally on (±)-56a had been prepared via the similar synthetic methodology. Among them, 56b with a methylenedioxy group on every benzene rings was found to exhibit most likely a very powerful neurite outgrowth promotion.
In addition, 56a and 56b induced regeneration of the mouse optic nerve in vivo, and their train was bigger than that of talaumidin, along with their in vitro measured train. Furthermore, the structure-activity relationship of 56b indicated that the two benzene rings had been essential constructions, and that the methyl groups on the THF ring would possibly enhance the neurotrophic train.
This finish consequence implies that the two benzene rings of the talaumidin derivatives are essential constructions for neurotrophic train, whereas the two methyl groups on the THF ring can enhance neurite-outgrowth train. Finally, it was observed that 1 and derivatives 56a and 56b exhibited potent regenerative train throughout the injured mouse optic nerve in vivo.
Reactants, merchandise, and transition states of elementary chemical reactions based totally on quantum chemistry.
Reaction cases, activation energies, branching ratios, yields, and many alternative quantitative attributes are important for actual pure syntheses and producing detailed response mechanisms. Often, it may very well be useful to have the power to categorise proposed reactions as fast or sluggish. However, quantitative chemical response data, notably for atom-mapped reactions, are troublesome to look out in current databases.
Therefore, we used automated potential vitality ground exploration to generate 12,000 pure reactions involving H, C, N, and O atoms calculated on the ωB97X-D3/def2-TZVP quantum chemistry diploma. We report the outcomes of geometry optimizations and frequency calculations for reactants, merchandise, and transition states of all reactions.
Additionally, we extracted atom-mapped response SMILES, activation energies, and enthalpies of response. We think about that this information will velocity up progress in automated methods for pure synthesis and response mechanism generation-for occasion, by enabling the occasion of novel machine learning fashions for quantitative response prediction.
