Abstract
The maintenance and manipulation of large genomes of DNA and RNA viruses had presented an obstacle to virological research. BAC vectors provided a solution to both problems as they can harbour large DNA sequences and can be efficiently modified using well-established mutagenesis techniques in Escherichia coli. Numerous herpesvirus and poxvirus DNA virus genomes were cloned into mini-F vectors.
Furthermore, several reverse genetic systems could be established for RNA viruses, such as members of Coronaviridae and Flaviviridae, based on BAC constructs. Transfection into susceptible eukaryotic cells of cloned viral DNA such as BAC allows reconstitution of recombinant viruses and bacteria. In this paper, we provide an overview of strategies that can be used for the generation of BAC vectors from viruses and also on systems that are currently available for various virus species.
In addition, we address common mutagenesis techniques that allow modification of BACs from single nucleotide substitutions to deletion of viral genes or insertion of foreign sequences. Finally, we review the reconstitution of viruses from BAC vectors and the removal of bacterial sequences from the virus genome during this process.
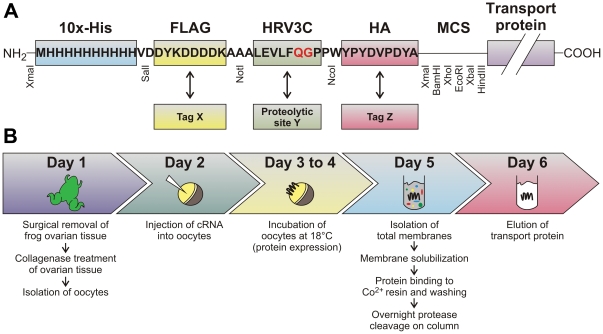
Cosmid-based approach
An alternative strategy often used for the generation of BACs from cell-associated viruses uses cosmid vectors to initially maintain overlapping parts of the DNA virus genome. The mini-F is subsequently inserted into one of the cosmids by ligation or homologous recombination in E. coli. Transfection of the overlapping cosmids into eukaryotic cells results in recombination between homologous sequences and reconstitution of infectious viruses.
During the process, the cosmid containing the mini-F cassette is incorporated into the virus genome, all resulting viruses harbour mini-F, and no laborious selection steps are required to obtain recombinant clones. As described above, circular virus DNA is isolated and transformed into E. coli and clones are analyzed for the integrity of the virus genomes they contain.
In vitro ligation
Recently, it has been shown that the mini-F replicon can be inserted into herpesvirus genomes by direct ligation. For this purpose, concatemeric virus DNA is isolated from herpes virus-infected cells and cleaved with a restriction enzyme that cuts only at a single locus within the virus genome. The resulting full-length viral genome is then ligated with a linearized mini-F vector containing compatible DNA ends. To prevent ligation of mini-F with cellular fragments, restriction enzymes that recognize an interrupted palindrome and allow the generation of desired directional sticky ends such as SfiI or BstXI can be used.
This strategy has been successfully applied to the generation of a BAC system for human herpesvirus 6A (HHV-6A). There are, however, several drawbacks to this method. First, the strategy requires a fully sequenced virus genome to determine potential restriction sites that can be used for the ligation procedure. Second, many virus genomes do not possess a single restriction site that is suitable for the approach.
Third, the mini-F insertion site is limited to the location of the single restriction site. Insertion into open reading frames (ORFs) or promoters of the virus genome can affect or abrogate the infectivity of BAC-derived viruses. Last but not least, the ligation and transformation procedures for large BAC vectors are very inefficient, making cloning attempts difficult.
Poxvirus Strategy
As described in Section 2.1, the cellular recombination machinery in mammalian cells can facilitate the insertion of mini-F sequences into the poxvirus genome. However, unlike herpesviruses, poxviruses do not produce a circular shape of the virus genome during replication. This poses a major obstacle to the transfer of recombinant poxvirus constructs to E. coli. To overcome the problem, infected cells are treated with isatin-β-thiosemicarbazone which promotes the accumulation of unresolved genomic concatemers.
For the generation of some poxvirus BAC clones, it was sufficient to transform E. coli with concatemeric DNA, a procedure that probably resulted in a recombination event that allowed circularization of the replicon. Alternatively, the isolated poxvirus DNA was circularized prior to E. coli transformation using the Cre/loxP or Flp/FRT recombination system.
Conclusions
Since the establishment of the first BAC system in 1997, BAC technology has contributed substantially to our understanding of the life cycle of large DNA and RNA viruses. Various techniques have been developed that facilitate the insertion of mini-F sequences into the virus genome. The methods enabled the generation of BAC systems for a plethora of virus species, including members of the Herpesvirales, Poxviridae, Coronaviridae, and Flaviviridae. The well-established mutagenesis techniques described herein facilitate site-specific manipulation of the virus genome in E. coli.
Various strategies can be used to introduce any desired modification, including deletions of viral sequences or insertions of foreign sequences. Reconstitution of recombinant viruses can be achieved by transfecting purified BAC DNA into susceptible mammalian cells, while in some cases additional helper viruses or expression vectors are required in this process. Finally, several techniques have been established that allow the excision of mini-F sequences from virus genomes without leaving behind an unwanted sequence.